Significant Functions of Fibroblast Growth Factor-10: A Narrative Review.
1. INTRODUCTION
Fibroblast growth factors (FGF) were first detected as mitogens in pituitary extracts [1] and its family consists of 22 members in mice and in humans [2]. This protein family participates in cell proliferation, differentiation, motility, and survival [1]. FGF/FGFR (Fibroblast Growth Factor Receptor) signaling pathway dysregulation is related to both developmental problems and cancer [3]. During development, FGFs have an important role in morphogenesis by controlling cellular proliferation, differentiation, and migration, while in adults, they act as homeostatic factors involved in tissue repair, regulation of the nervous system, and tumor angiogenesis [4].
Fibroblast growth factor-10 (FGF-10) is a secreted glycoprotein whose effects are applied through two fibroblast growth factor receptors including FGFR1B and FGFR2B, with low and high affinity, respectively [5]. This growth factor is a mesodermally derived mitogen which contributes to epithelial-mesenchymal interaction (EMI) during embryogenesis [6]. The crucial role of EMI in the organogenesis of several organs is well documented [7]. FGF-10 is expressed in the mesenchyme and exerts its influence in a paracrine mode in the epithelium [8]. The signaling pathways of FGF-10 are PLCγ, PI3K–AKT, and Ras/MAPK [8]. The PLCγ signaling pathway affects migration, adhesion, and cell morphology [9]. The PI3K-AKT signaling pathway also adjusts cell survival and fate determination, while the Ras/MAPK signaling pathway regulates cell proliferation and differentiation [9].
Fluorescence in situ hybridization (FISH) analysis was performed in order to identify the location of the FGF-10 gene and the result mapped the FGF-10 gene in to the 5p12-p13 locus of chromosome 5 [10]. The isolated cDNA of FGF-10 from human lungs encodes a protein that consists of 208 amino acids [11] weighing 19kD [12], with a high sequence similar to rat FGF-10 (95.6%) [11]. In addition, a hydrophobic plot analysis indicated that both the human and the rat FGF-10 possessed the hydrophobic N-terminal including 40 amino acids that possibly act as a signal peptide [11]. This review presents the importance of FGF-10’s different functions in terms of development, disease, wound healing, and therapeutic value.
2. FGF-10 AND DEVELOPMENT
The analysis of the results of Fgf10 and Fgfr2b mutant mice and null mice show that the FGF10/FGFR2b signaling pathway has a significant role in the development of branching organs such as the pancreas, mammary gland, salivary gland, thyroid gland, and lungs [13]. This is supported by reports of mice with Fgf10 deficiency showing various tissue defects in the lungs, limbs, and mammary glands [14]. FGF signaling is also involved in neural crest induction which leads to the formation of the central nervous system and future epidermis [15]. Blocking FGF signaling in the explant prevents the induction of neural crest in response to paraxial mesoderm signals [16]. Within tongue skeletal muscle morphogenesis, TGF-β induced FGF-10 signaling in cranial neural crest plays a vital role in myogenic progenitor development via the adjustment of tissue-tissue interactions [17]. Also, a special pattern of FGF-10 expression in the adult mammalian brain adjusts neurogenesis and preserves neurogenic potential [18]. By regulating well-timed differentiation of radial glia, transient Fgf10 expression controls the length of the primary progenitor expansion stage, initiation of neurogenesis, and the number of progenitors and neurons fated to specific cortical areas [19]. Fgf10 also acts as a key controller of the neuroepithelial to radial glial transition and the subsequent rostral forebrain size [20].
Table 1 demonstrates the importance of FGF-10 functions in the development of several organs.
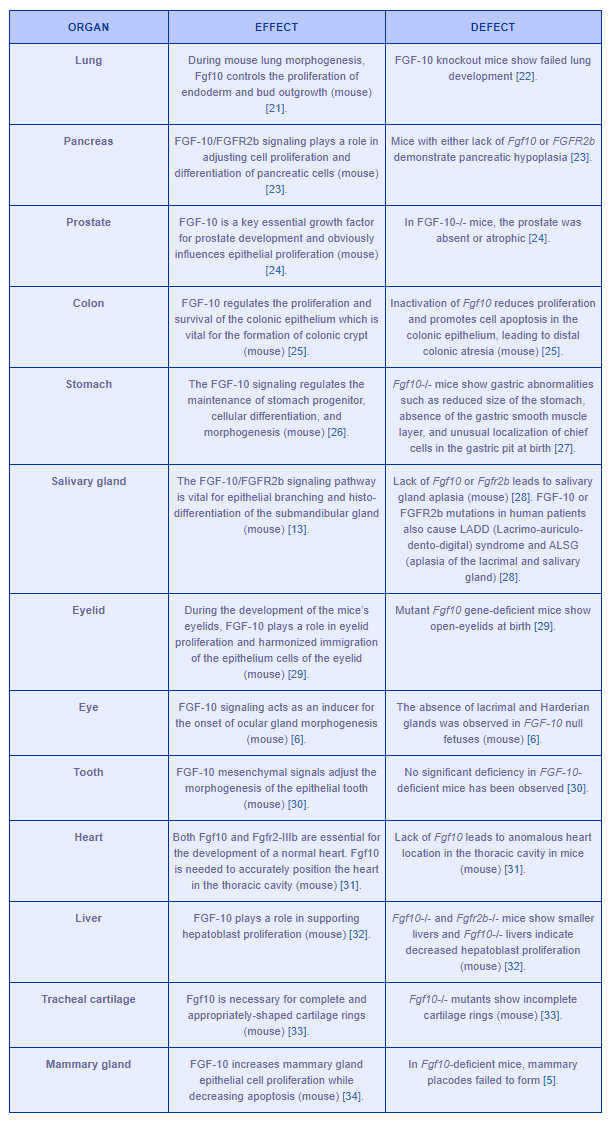
Table 1: FGF-10 key roles in the development of several organs. Difficulties in either FGF-10 or FGFR2IIIb lead to anatomical and physiological anomalies.
3. FGF-10 IN CANCER AND EPITHELIAL-MESENCHYMAL TRANSITION (EMT)
FGF-10 has an important role in the proliferation of cancer cells by enhancing the transition of G1 to S phase and thus preparing the cells for mitosis [36]. By applying immunohistochemistry and in situ hybridization, mRNA of FGF-10 was seen in both colorectal cancer cells and adjacent fibroblast of cancer cells [37]. There was no detection of FGF-10 mRNA in normal colorectal tissues which indicates the possible role of FGF-10 in the growth of colorectal adenocarcinoma cells [37]. One in vivo study has shown that overexpression of the FGF-10 gene stimulates the formation of organized tumors with type II epithelial cell features in the lung [38]. Also, a study on human breast carcinomas has shown that Fgf10 mRNA levels have been strongly enhanced in approximately 10% of the tested human breast tumors, proposing that Fgf10 may play a role in the oncogenicity of a subdivision of human breast cancers [39]. FGF-10 has an important role in some other cancers such as pancreatic cancer (the FGF-10/FGF2IIIb signaling pathway stimulates cell immigration and invasion in pancreatic cancer) [40] and prostate cancer (an increase in mesenchymal FGF-10 expression promotes formation of well differentiated prostate cancer) [41].
FGF-10 is also known as a significant factor during type-I EMT (happens during embryogenesis) and it is also important in type-III EMT (happens during cancer and metastasis) [36]. EMT is considered a process in which epithelial cells lose their connections and acquire the ability to migrate freely and adopt mesenchymal phenotype [9]. EMT happens during embryonic progression, as a physiological response during wound healing, and is also essential for the development of cancer [42]. In fact, it is a significant process in cancer metastasis, interrupting cell-cell adhesion and enhancing tumor cell invasion [43]. FGF-10 is an important factor during both type-I and III EMT [36]. FGF-10 may stimulate EMT via Ras/MAPK and AKT/PI3K/mTOR signaling pathways [36]. The PI3K/AKT signaling pathway is necessary for FGF-induced EMT and cell survival [44]. FGF-10 expression reduces gene expression of E-cadherin (responsible for epithelial adherence connections) while increasing Slug (SNAI2) and Snail (SNAI1) (two transcription factors known as EMT- inducers) [36]. The loss of the E-cadherin expression is one of the important agents all throughout the EMT process in both development and cancer [45]. The lack of E-cadherin also enhances invasiveness of tumor cells in vitro, and it is associated with the transmission of adenoma to carcinoma in animal models [45]. In fact, the E-cadherin expression level is inversely correlated with the grade and stage of the tumor [45]. Moreover, up-regulation of FGF-10 promotes cell sensitivity to TGF-β [36]. TGF-β acts as a strong regulator of cellular adhesion by decreasing E-cadherin expression [46]. Overall, increased levels of TGF-β reduces adhesiveness and increases the motility of cancer cells [47].
4. FGF-10 AND DISEASES
FGF-10 encoding gene mutations are related to aplasia of the lacrimal and major salivary glands (ALSG), which is a rare and autosomal dominant disorder characterized by atresia, aplasia, and hypoplasia of the lacrimal and salivary glands [48]. ALSG displays symptoms such as epiphora, irritable eyes, recurring eye infections, oral inflammation, dental erosion, dental caries, and xerostomia [48]. Babies with ALSG show no apparent lung disorders [47]. However, mature patients with heterozygous lack of function of FGF-10 demonstrate a meaningful reduction in IVC (Inspiratory Vital Capacity), FEV1 (Forced Expiratory Volume in one second), and FEV1/IVC quota in comparison with the predicted reference values and non-carrier siblings [47]. Genetic variants that influence FGF-10 signaling are also important in determining lung function, which may ultimately have a role in chronic obstructive pulmonary disease [49]. Also, FGF-10 protein defect has been reported in patients with broncho-pulmonary dysplasia, a chronic lung disease in prematurely-born infants, which is one of the main causes of morbidity and mortality [50].
5. FGF-10 AND WOUND HEALING
FGF-10 is mainly generated by neuron and microglia / macrophages, and its expression is elevated after a spinal cord injury, especially after an acute phase, which suggests that FGF-10 can be considered as a potential treatment for injuries of the central nervous system [18]. Exogenous FGF-10 treatment leads to the improvement of functional recovery by decreasing apoptosis and also by repairing neurites through the FGFR2/PI3K/Akt signaling pathway [18]. The PI3K/Akt signaling pathway is vital for growth and survival in many biological processes such as early ischemia/reperfusion injury [18]. Moreover, in vivo FGF-10 treatment suppresses the activation of the NF-κB signaling pathway (the main inflammatory mediator that controls the production of TNF-α and IL-6 inflammatory factors in CNS) in the context of ischemic stroke, which results in decreased neuro-inflammation [51].
Preliminary in vitro wound healing examinations have indicated that FGF-10 increases the proliferation and differentiation of human keratinocytes, proposing a possible role of FGF-10 in epidermal growth and differentiation [12]. In fact, accelerated skin wound healing as well as re-epithelialization and formation of granulation tissue are among the known roles of FGF-10 [37]. FGF-10 is the most effective in wound healing compared with other growth factors such as FGF-7 (KGF) and TGF-β, which have been examined in the scar formation of rabbits [37]. In addition, FGFR2IIIb ligands, in particular FGF-10, are strongly expressed during skin wound healing and probably result in an increase in the amount of Nrf2 (a significant factor during cellular stress reply) during wound healing [52]. FGF-10 is also considered a strong inducer of wound healing because of its ability to boost mechanical strength via the enhancement of the amount of wound collagen and epidermal thickness [53]. In general, it has been suggested that FGF-10 acts as a potential wound healing factor for inducing epithelialization in several types of wounds, such as partial-thickness burn wounds, venous stasis ulcers, or skin graft donor sites [12].
6. FGF-10 AND THERAPEUTIC VALUE
The truncated form of FGF-10 that maintains the biological and physiological activity of full length FGF-10 is called ‘repifermin’ [54]. The application of repifermin has been shown as a potential therapeutic method to stimulate angiogenesis [54]. A randomized trial estimating the safety and clinical effects of repifermin was conducted on patients with mucositis treated with auto-HSCT (Autologous Hematopoietic Stem Cell transplantation). The results of phase I/II of the trial indicated that repifermin given before and after auto-HSCT is well tolerated and appears active in decreasing the incidence of mucositis. However, this result will need to be confirmed through larger randomized clinical studies [55]. In this placebo-controlled study, patients who received 50 g/kg of repifermin demonstrated a lower score of worst pain on swallowing as well as on eating capabilities and they also tended to use narcotic pain medication (given specifically for mucositis-related pain) for fewer days [55]. In a randomized, placebo-controlled and multicenter trial, repifermin was given to patients in order to evaluate its effect on chronic venous ulcers. Results suggest that it was also well tolerated and that it accelerated wound healing when compared with the placebo [56]. These results were more significant in patients with a primary wound zone less than 15cm2 and with wounds less than 18 months old [56]. Furthermore, repifermin does not promote proliferation of FGFR2IIIb (KGFR, Keratinocyte Growth Factor Receptor) positive human epithelial-like tumors (in vivo and in vitro), which underlines its potential to specifically target normal epithelial tissues. This suggests that medication can be developed for cancer patients suffering from chemo- or radiotherapy-induced mucositis, inflammatory bowel diseases, and other kinds of epithelial tissue damage [57].
7. CONCLUSION
FGF-10 signaling is important for cell proliferation, differentiation, survival, adhesion, and EMT: events which are all essential during both embryogenesis and the development of pathological conditions such as cancer. To determine how such a valuable developmental factor becomes active as a malignant factor in cancer and what different factors may be involved, further basic molecular research would be worthwhile. In other words, a true understanding of the FGF-10 signaling mechanism and its significant role during embryogenesis and EMT may suggest a new candidate for a new, targeted cancer therapy. Furthermore, deeper insights into the reparative functions of FGF 10 may have significant implications in the fields of regenerative medicine and wound care.
8. REFERENCES
[1] Martin, G.R., The roles of FGFs in the early development of vertebrate limbs. Genes & development, 1998. 12(11): p. 1571-1586.
[2] Murakami, M., A. Elfenbein, and M. Simons, Non-canonical fibroblast growth factor signalling in angiogenesis. Cardiovascular research, 2007. 78(2): p. 223-231.
[3] Brooks, A.N., E. Kilgour, and P.D. Smith, Fibroblast growth factor signaling: A new therapeutic opportunity in cancer. Clinical cancer research, 2012: p. clincanres. 0699.2012.
[4] Cotton, L.M., M.K. O’Bryan, and B.T. Hinton, Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocrine reviews, 2008. 29(2): p. 193-216.
[5] Chioni, A.-M. and R. Grose, Negative regulation of fibroblast growth factor 10 (FGF-10) by polyoma enhancer activator 3 (PEA3). European journal of cell biology, 2009. 88(7): p. 371-384.
[6] Govindarajan, V., et al., Endogenous and ectopic gland induction by FGF-10. Developmental biology, 2000. 225(1): p. 188-200.
[7] Botchkarev, V.A. and J. Kishimoto. Molecular control of epithelial–mesenchymal interactions during hair follicle cycling. in Journal of Investigative Dermatology Symposium Proceedings. 2003. Elsevier.
[8] Itoh, N., FGF10: A multifunctional mesenchymal–epithelial signaling growth factor in development, health, and disease. Cytokine & growth factor reviews, 2016. 28: p. 63-69.
[9] Teven, C.M., et al., Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes & diseases, 2014. 1(2): p. 199-213.
[10] Bagai, S., et al., Fibroblast growth factor-10 is a mitogen for urothelial cells. Journal of Biological Chemistry, 2002. 277(26): p. 23828-23837.
[11] Emoto, H., et al., Structure and expression of human fibroblast growth factor-10. Journal of Biological Chemistry, 1997. 272(37): p. 23191-23194.
[12] Plichta, J.K. and K.A. Radek, Sugar-coating wound repair: a review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. Journal of Burn Care & Research, 2012. 33(3): p. 299-310.
[13] Jaskoll, T., et al., FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC developmental biology, 2005. 5(1): p. 11.
[14] Burns, R., et al., Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Developmental biology, 2004. 265(1): p. 61-74.
[15] Mikrut, S.D.O., The Role of the LIM Adaptors LMO4 and Ajuba LIM in Regulating Snail Family Mediated Neural Crest Development, 2013, Northwestern University.
[16] Heeg-Truesdell, E. and C. LaBonne, A slug, a fox, a pair of sox: transcriptional responses to neural crest inducing signals. Birth Defects Research Part C: Embryo Today: Reviews, 2004. 72(2): p. 124-139.
[17] Hosokawa, R., et al., TGF-β-mediated FGF10 signaling in cranial neural crest cells controls development of myogenic progenitor cells through tissue–tissue interactions during tongue morphogenesis. Developmental biology, 2010. 341(1): p. 186-195.
[18] Chen, J., et al., Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell death & disease, 2017. 8(10): p. e3090.
[19] Sahara, S. and D.D. O’Leary, Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron, 2009. 63(1): p. 48-62.
[20] Phoenix, T.N. and S. Temple, Baby got brain: FGF10 sets rostral cortical size. Neuron, 2009. 63(1): p. 1-3.
[21] Bellusci, S., et al., Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development, 1997. 124(23): p. 4867-4878.
[22] Beenken, A. and M. Mohammadi, The FGF family: biology, pathophysiology and therapy. Nature reviews Drug discovery, 2009. 8(3): p. 235.
[23] Hart, A., S. Papadopoulou, and H. Edlund, Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Developmental dynamics: an official publication of the American Association of Anatomists, 2003. 228(2): p. 185-193.
[24] Donjacour, A.A., A.A. Thomson, and G.R. Cunha, FGF-10 plays an essential role in the growth of the fetal prostate. Developmental biology, 2003. 261(1): p. 39-54.
[25] Sala, F.G., et al., Fibroblast growth factor 10 is required for survival and proliferation but not differentiation of intestinal epithelial progenitor cells during murine colon development. Developmental biology, 2006. 299(2): p. 373-385.
[26] Nyeng, P., et al., FGF10 signaling controls stomach morphogenesis. Developmental biology, 2007. 303(1): p. 295-310.
[27] Spencer–Dene, B., et al., Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b–mediated signaling. Gastroenterology, 2006. 130(4): p. 1233-1244.
[28] Wells, K.L., et al., Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biology open, 2013: p. BIO20135306.
[29] Tao, H., et al., A dual role of FGF10 in proliferation and coordinated migration of epithelial leading edge cells during mouse eyelid development. Development, 2005. 132(14): p. 3217-3230.
[30] Kettunen, P., et al., Associations of FGF‐3 and FGF‐10 with signaling networks regulating tooth morphogenesis. Developmental dynamics: an official publication of the American Association of Anatomists, 2000. 219(3): p. 322-332.
[31] Marguerie, A., et al., Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovascular research, 2006. 71(1): p. 50-60.
[32] Berg, T., et al., Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via β-catenin activation. Hepatology, 2007. 46(4): p. 1187-1197.
[33] Sala, F.G., et al., FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development, 2011. 138(2): p. 273-282.
[34] Cui, Y. and Q. Li, Expression and functions of fibroblast growth factor 10 in the mouse mammary gland. International journal of molecular sciences, 2013. 14(2): p. 4094-4105.
[35] Lu, P., et al., Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Developmental biology, 2008. 321(1): p. 77-87.
[36] Abolhassani, A., et al., FGF10: type III epithelial mesenchymal transition and invasion in breast cancer cell lines. Journal of Cancer, 2014. 5(7): p. 537.
[37] Matsuike, A., et al., Expression of fibroblast growth factor (FGF)-10 in human colorectal adenocarcinoma cells. Journal of Nippon Medical School, 2001. 68(5): p. 397-404.
[38] Clark, J.C., et al., FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2001. 280(4): p. L705-L715.
[39] Theodorou, V., et al., Fgf10 is an oncogene activated by MMTV insertional mutagenesis in mouse mammary tumors and overexpressed in a subset of human breast carcinomas. Oncogene, 2004. 23(36): p. 6047.
[40] Nomura, S., et al., FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. British journal of cancer, 2008. 99(2): p. 305.
[41] Memarzadeh, S., et al., Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer cell, 2007. 12(6): p. 572-585.
[42] Thiery, J.P., et al., Epithelial-mesenchymal transitions in development and disease. cell, 2009. 139(5): p. 871-890.
[43] Turner, N. and R. Grose, Fibroblast growth factor signalling: from development to cancer. Nature Reviews Cancer, 2010. 10(2): p. 116.
[44] Katoh, M. and M. Katoh, Cross-talk of WNT and FGF signaling pathways at GSK3β to regulate β-catenin and SNAIL signaling cascades. Cancer biology & therapy, 2006. 5(9): p. 1059-1064.
[45] Kang, Y. and J. Massagué, Epithelial-mesenchymal transitions: twist in development and metastasis. cell, 2004. 118(3): p. 277-279.
[46] Elliott, R.L. and G.C. Blobe, Role of transforming growth factor Beta in human cancer. Journal of clinical oncology, 2005. 2(9): p.23 2078-2093.
[47] Chao, C.-M., et al., Alveologenesis: key cellular players and fibroblast growth factor 10 signaling. Molecular and cellular pediatrics, 2016. 3(1): p. 17.
[48] Chapman, D.B., V. Shashi, and D.J. Kirse, Case report: aplasia of the lacrimal and major salivary glands (ALSG). International journal of pediatric otorhinolaryngology, 2009. 73(6): p. 899-901.
[49] Klar, J., et al., Fibroblast growth factor 10 haploinsufficiency causes chronic obstructive pulmonary disease. Journal of medical genetics, 2011. 48(10): p. 705-709.
[50] Chao, C.M., et al., Fgf10 deficiency is causative for lethality in a mouse model of bronchopulmonary dysplasia. The Journal of pathology, 2017. 241(1): p. 91-103.
[51] Li, Y.-H., et al., Neuron-derived FGF10 ameliorates cerebral ischemia injury via inhibiting NF-κB-dependent neuroinflammation and activating PI3K/Akt survival signaling pathway in mice. Scientific reports, 2016. 6: p. 19869.
[52] Braun, S., et al., Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Molecular and cellular biology, 2002. 22(15): p. 5492-5505.
[53] Jimenez, P.A. and M.A. Rampy, Keratinocyte growth factor-2 accelerates wound healing in incisional wounds. Journal of Surgical Research, 1999. 81(2): p. 238-242.
[54] Huang, M. and C. Berkland, Controlled release of Repifermin\textsuperscript{\textregistered from polyelectrolyte complexes stimulates endothelial cell proliferation}. Journal of pharmaceutical sciences, 2009. 98(1): p. 268-280.
[55] Freytes, C.O., et al., Phase I/II randomized trial evaluating the safety and clinical effects of repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clinical cancer research, 2004. 10(24): p. 8318-8324.
[56] Robson, M.C., et al., Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor-2) to accelerate wound healing in venous ulcers. Wound repair and regeneration, 2001. 9(5): p. 347-352.
[57] Alderson, R., et al., In vitro and in vivo effects of repifermin (keratinocyte growth factor-2, KGF-2) on human carcinoma cells. Cancer chemotherapy and pharmacology, 2002. 50(3): p. 202-212.
Article photo credit: ZEISS Microscopy
- Log in to post comments










